Metabolism & Fat
In mammals, adipose tissue serves a pivotal role as the primary source for calories when energy intake is insufficient to meet the metabolic demands. Efficient storage and release of substrates (fatty acids and glycerol) from fat are critical for normal metabolic function. Fat sits as the crossroads of many biological systems and contributes to various common diseases. When adipocyte function is reduced, as occurs in obesity or lipodystrophies, lipid accumulates in non-adipocytes and impairs the normal function of liver, skeletal and heart muscle, the pulmonary tree and the vascular system - contributing to type 2 diabetes, cardiomyopathies, non-alcoholic fatty liver disease and asthma. Obesity and adipocyte dysfunction also contribute, in ways that are not understood, to the development of cancers, hypertension, dementia and autoimmune disorders. The epidemic of obesity that is occurring throughout the world makes understanding the development, function and regulation of adipose tissue a pressing public health challenge. It is also a really cool tissue that allows us to study behavior, immunology, cell biology and metabolism.
from Cell Metabolism (2014) 20:565-72
Neuroendocrine Systems that Regulate Fat Mass - One Yet to be Discovered
Mammals regulate their fat mass so attempts to increase or decrease one's weight are met with neuroendocrine responses that favor a return to baseline weight. Leptin is a hormone secreted by adipocytes that is a critical signal that protects agains weight loss. Restricting food intake and reducing fat mass, lowers the production and circulating concentration of leptin. This lower leptin concentration signals feeding and metabolic regulatory centers of the brain to increase food seeking behavior, eating and energy efficiency - response that work to restore one's body weight.
When leptin was identified in 1994, it established that adipose tissue is an endocrine organ that regulates its own mass and thereby helps maintain energy homeostasis. It was hoped that higher concentrations of leptin, acting in a manner similar to other neuroendocrine systems, would activate a counter regulator response and reduce feeding behavior and lower energy efficiency. It was hoped that leptin would be a therapy to treat common forms of obesity. Unfortunately, leptin has proved ineffective, even a pharmacological doses, to reduce weight in humans. This led many to invoke "leptin resistance," as a reason for leptin in ability to elicit a response seen during overfeeding. However, we and others have hypothesized that a system distinct of leptin is responsible for the defense of increased body weight. We recently demonstrated that mice will defend against increased body fat by reducing food intake by a mechanism that does depend upon increased leptin concentration.
Live image of macrophages in adipose tissue - from Xiaoyuan Xu
The Immunology of Metabolism
For more than half-a-century obesity was known to increase circulating concentration of inflammatory molecules and in the 1990's several groups found that the expansion of fat mass induces inflammation of adipose tissue directly. A decade and half ago, ours and another lab discovered that obesity increases the macrophage content of adipose tissue so that in the most obese individuals and rodents more than 50% of the cells in a fat depot are immune cells. These findings have since been expanded to demonstrate that the metabolic state of a tissue leads to the expansion and alteration of many immune populations not only macrophages and not only in adipose tissue but in liver, bone marrow, brain, pancreas, lungs and other organs.
Many questions remain. Perhaps the most compelling are what drives the immune response to changes in metabolic state, and what roles, both adaptive and maladaptive, do immune cells play metabolically active tissues. We have hypothesized that the immune system, and macrophages in particular, play a key roles in the development and lipid homeostasis of adipose tissue. Work in our laboratory currently focuses on identifying the ontogeny of sub-populations of macrophages and their specific roles. Our studies and the work of others suggest that distinct populations drive hypertrophy and expansion of adipose tissue, while other are critical in the turn over of lipid in fat and the maintenance of healthy adipose tissue.
Novel Lipid Particles
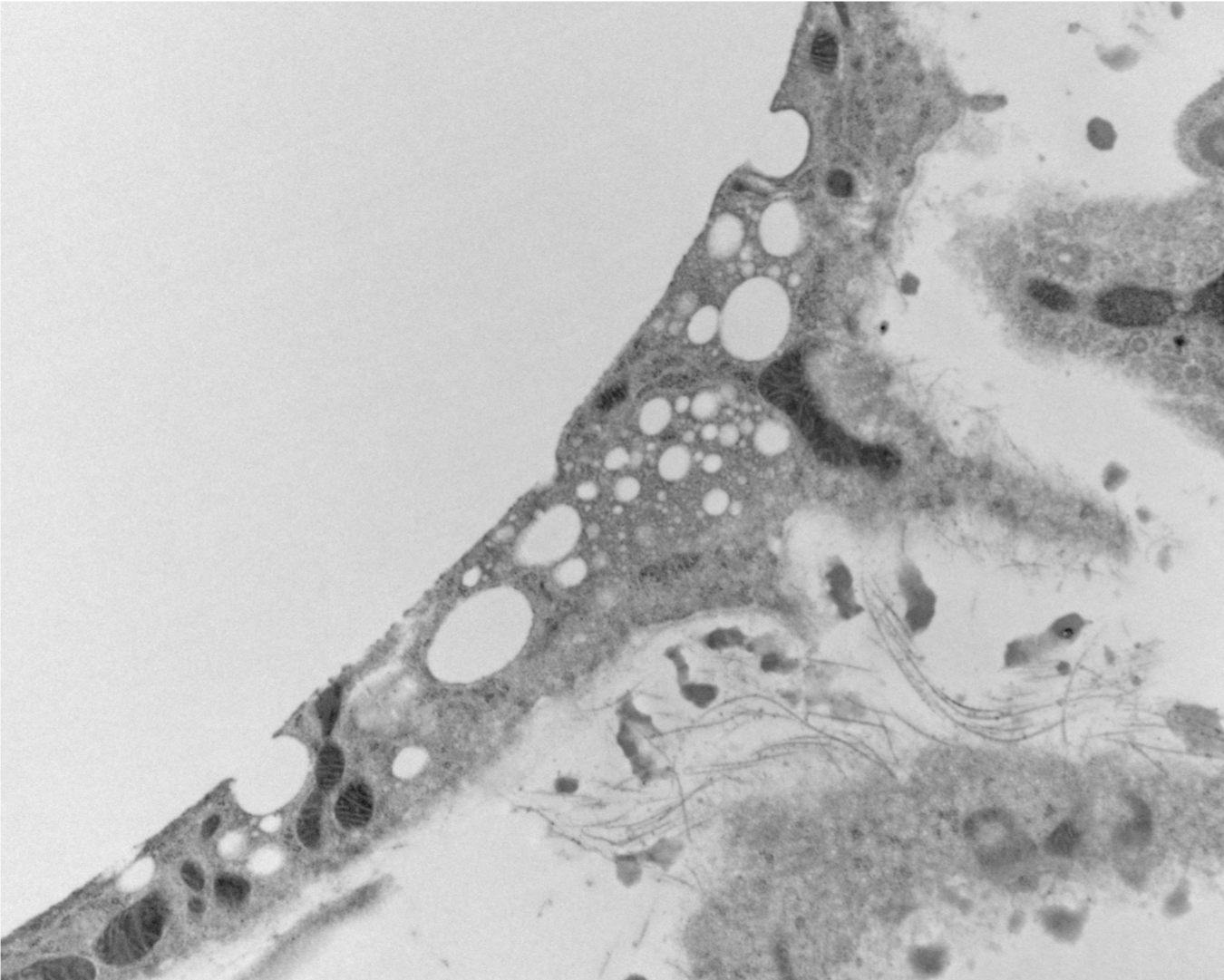
As we studied the accumulation of lipid in immune cells of adipose tissue we were puzzled as to how the lipid got into these cells. We had incorrectly assumed that the normal breakdown products of fat - fatty acids and glycerol - we scavenged by macrophages. But a series of experiment suggested that lipid was taken up whole as triglycerides. This lead up to discover that adipocytes release lipid not only by the canonical pathway of lipolysis but also by an entire novel pathway of lipid particle release. These extracellular lipid-filled vesicles are produced at an astonishing rate so that the entire lipid in a fat cell would turnover in 50-100 days - changing our view of fat as a long-term storage depot. We are only now beginning to unravel some of the basic biology of this process and trying to understand its implications.
Lysosome Function in Metabolism
It seems counterintuitive, but lysosome breakdown of lipid is crucial for adipose tissue to maintain its mass. Without the normal ability to degrade triglycerides and other neutral lipids, fat in mice and humans with genetic mutations lose their fat overtime. This has been long known but poorly understood. We've recently discovered that adipose tissue macrophages activate a program of lysosome degradation of lipids that establishes a local cycle of lipid release from fat cells and breakdown by macrophages. This lysosomal proposes also appears to help drive the tissue specific differentiation of macrophages in adipose tissue.